
Chemistry : Shapes Of Molecules Electron Repulsion Theory
325.89AED
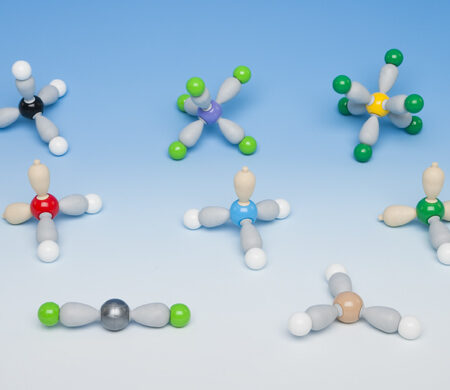
His set contains sufficient parts to make eight atomic models. Each model is an example of the different orientations of the bonds and cover co-ordination numbers 1 to 6. Lone pairs are represented by brown spheres or brown pear shaped parts. The latter is useful as protonated models of NH4+ and H3O+ may be built thus demonstrating Acid/Base theory.

Chemistry : Shapes Of Molecules Electron Repulsion Theory
325.89AED
- Description
- Reviews (0)
Description
His set contains sufficient parts to make eight atomic models. Each model is an example of the different orientations of the bonds and cover co-ordination numbers 1 to 6. Lone pairs are represented by brown spheres or brown pear shaped parts. The latter is useful as protonated models of NH4+ and H3O+ may be built thus demonstrating Acid/Base theory.
Spheres: 1 carbon (4 holes), 1 oxygen (4 holes), 1 nitrogen (4 holes), 1 sulphur (6 holes), 1 phosphoros (5 holes), 1 chlorine (4 holes), 1 boron (3 holes), 1 metal (beryllium) (2 holes), 7 chlorine (1 holes), 9 fluorine (1 holes), 13 hydrogen, 6 circular lone pair orbitals, 6 pear shaped lone pair orbitals. Links: 26 grey pear shaped sigma bonds, 6 short links (to be used with circular lone pairs).
Be the first to review “Chemistry : Shapes Of Molecules Electron Repulsion Theory” Cancel reply
Related Products
-
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, SENSORY PLAY & GLITTER PANEL
EASY HOLD PURPLE GLITTER PANEL-COM
150.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, SENSORY PLAY & GLITTER PANEL
EASY HOLD GOLD GLITTER PANEL-COM
150.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES
EASY HOLD GLOW PANELS SET-COM
437.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, WOODEN & MIRROR
EASY HOLD GLOW PANEL ORANGE-COM
150.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, WOODEN & MIRROR
EASY HOLD GLOW PANEL GREEN-COM
150.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, SENSORY PLAY & GLITTER PANEL
SENSORY MOOD CUBE-COM
1,625.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, SENSORY PLAY & GLITTER PANEL
SENSORY MOOD BALL-COM
1,275.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, SENSORY PLAY & GLITTER PANEL
SENSORY MOOD EGG-COM
1,200.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, WOODEN & MIRROR
WOODEN DISCOVERY DIVIDERS-COM
207.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, SENSORY PLAY & GLITTER PANEL
SENSORY MOOD DISCOVERY TABLE-COM
3,657.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
TRANSLUCENT STACKABLE COUNTERS-COM
345.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
TRANSLUCENT MODULE BLOCKS-COM
482.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
TRANSLUCENT COLOUR BLOCKS-COM
620.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
EARLY YEARS MATHS RESOURCE SET-COM
1,000.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
TRANSLUCENT SORTING BOWLS-COM
125.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES
TRANSLUCENT COLOUR BUCKETS-COM
200.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
TRANSLUCENT COLOUR POTS-COM
247.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
TRANSLUCENT COLOUR JUGS-COM
137.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
TRANSLUCENT COLOUR TWEEZERS-COM
220.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
TRANSLUCENT COLOUR PADDLES-COM
62.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
SPLATS-COM
282.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
COLOUR ACETATE SHEETS-COM
107.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
PERCEPTION SEMISPHERES-COM
562.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
COLOUR CRYSTAL BLOCK SET-COM
962.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES
WOODEN PLAY TABLE-COM
757.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
A2 LIGHT PANEL & COVER-COM
2,600.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
A3 LIGHT PANEL-COM
1,137.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
A3 EXPLORATION LIGHT TRAY-COM
975.00AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
A3 LIGHT PANEL & EXPLORATION TRAY-COM
2,112.50AED0 out of 5There is no AI review summary. -
EARLY YEARS PRIMARY & SECONDARY, EDUCATIONAL RESOURCES, LIGHT & COLOUR PLAY
ROUND LIGHT PANEL 60CM-COM
1,787.50AED0 out of 5There is no AI review summary.







































































Reviews
There are no reviews yet.